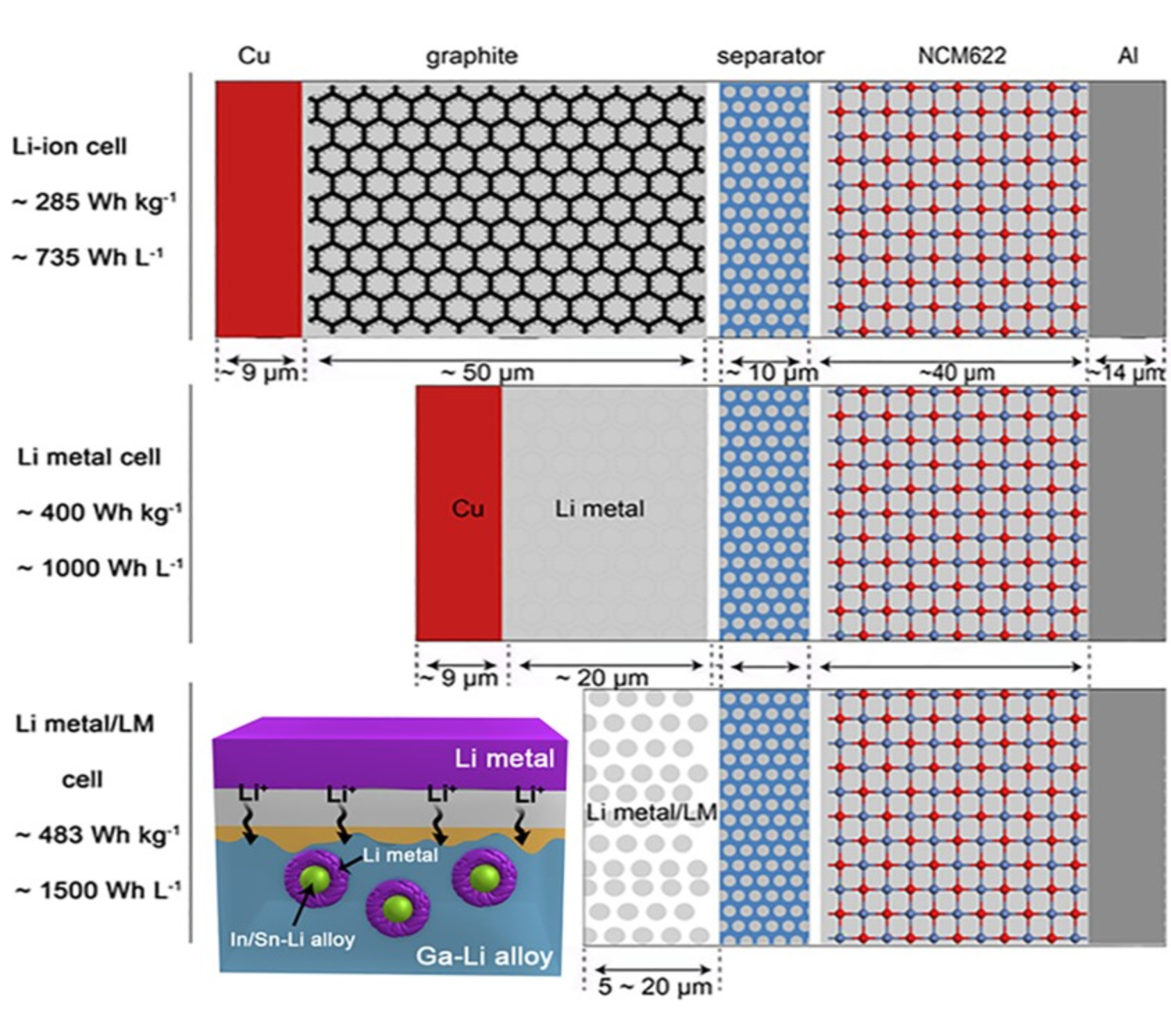
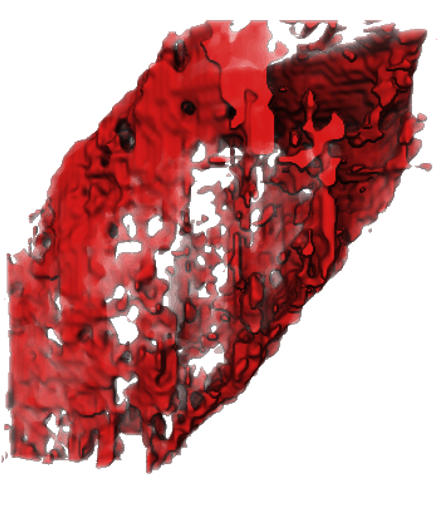
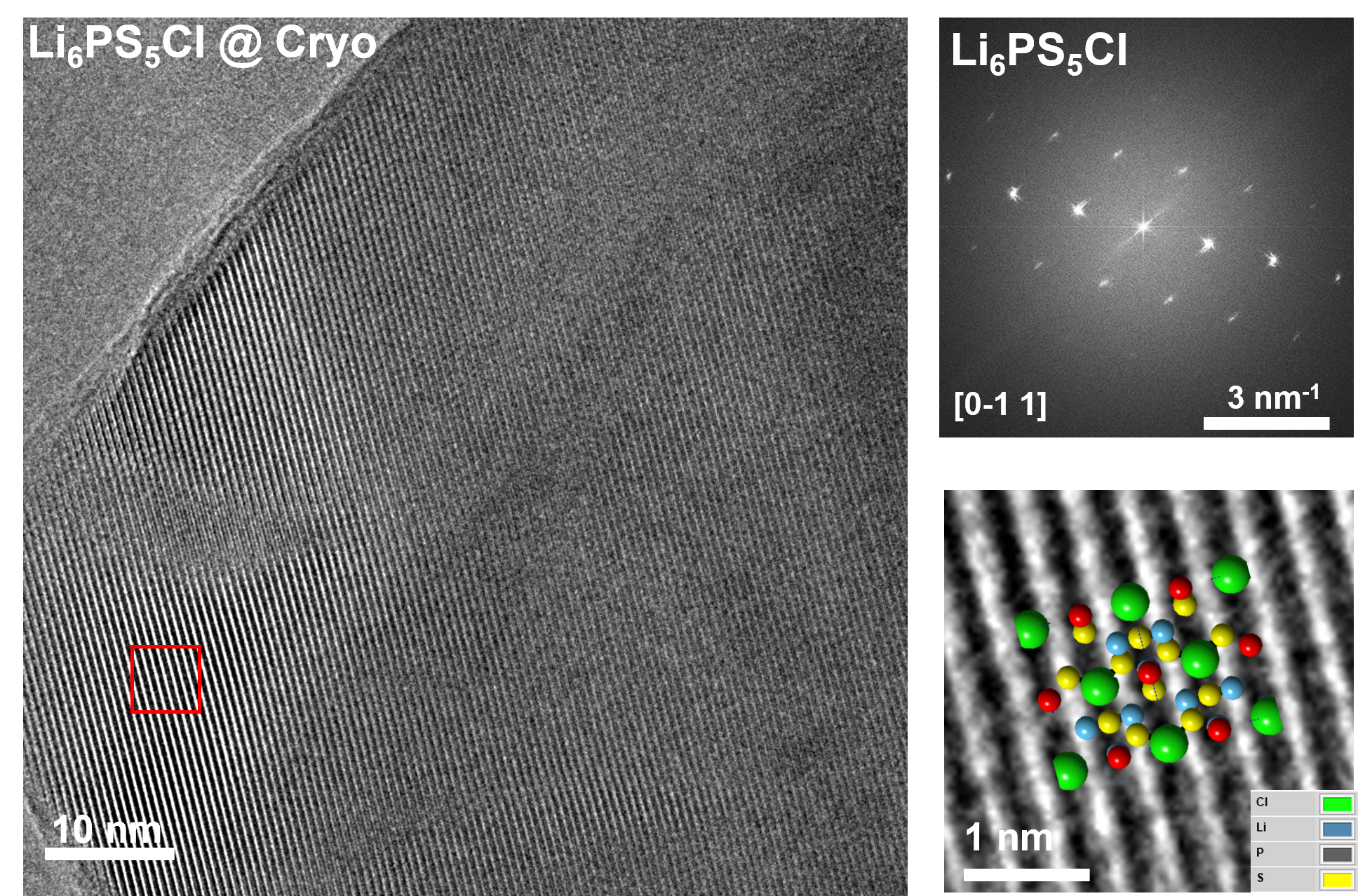
Analyzing Li metal battery using low dose Cryo-HRTEM and Cryo-EELS Tomography

Using cryogenic transmission electron microscopy, we revealed three-dimensional (3D) structural details of the electrochemically plated lithium (Li) flakes and their solid electrolyte interphase (SEI). As the SEI skin layer is largely composed of nanocrystalline LiF and Li2O in amorphous polymeric matrix, when complete Li stripping occurs, the compromised SEI 3D framework buckles and wrinkles. The flexibility and resilience of the SEI skin layer plays a vital role in preserving an intact SEI 3D framework after Li stripping. The intact SEI network enables the nucleation and growth of newly plated Li inside the previously formed SEI network in subsequent cycles, preventing additional large amounts of SEI formation. In addition, cells cycled under the accurately controlled uniaxial pressure can further enhance the repeated utilization of the SEI and improve the Coulombic efficiency (CE) by up to 97%, demonstrating an effective strategy for reducing the formation of additional SEI and inactive “dead” Li.